Tesofensine Anti-obesity Medicine
페이지 정보
작성자 Luis Mincey 작성일24-12-16 05:59 조회16회관련링크
본문
 Research study into obesity therapy is expanding right into new areas, including the exploration of therapies for related metabolic conditions. This growth not just expands the extent of possible healing applications but also enhances our understanding of the intricate connections within metabolic health and wellness. The field International shipping of Tesofensine obesity therapy is seeing considerable innovations, particularly with the development of new pharmacological representatives like berberine.
Research study into obesity therapy is expanding right into new areas, including the exploration of therapies for related metabolic conditions. This growth not just expands the extent of possible healing applications but also enhances our understanding of the intricate connections within metabolic health and wellness. The field International shipping of Tesofensine obesity therapy is seeing considerable innovations, particularly with the development of new pharmacological representatives like berberine.Brand-new Cns Techniques For Treatment Of Weight Problems
You have actually likely listened to tales of people who have actually struggled with their weight for years, attempting various diet regimens and workout programs without success. For lots of, weight-loss drugs like Semaglutide and Tirzepatide supply an actual, lasting, science-backed technique to accomplishing their weight objectives. Nevertheless, since the FDA authorized Semaglutide for fat burning, the drug has actually been swarmed with countless myths and mistaken beliefs.
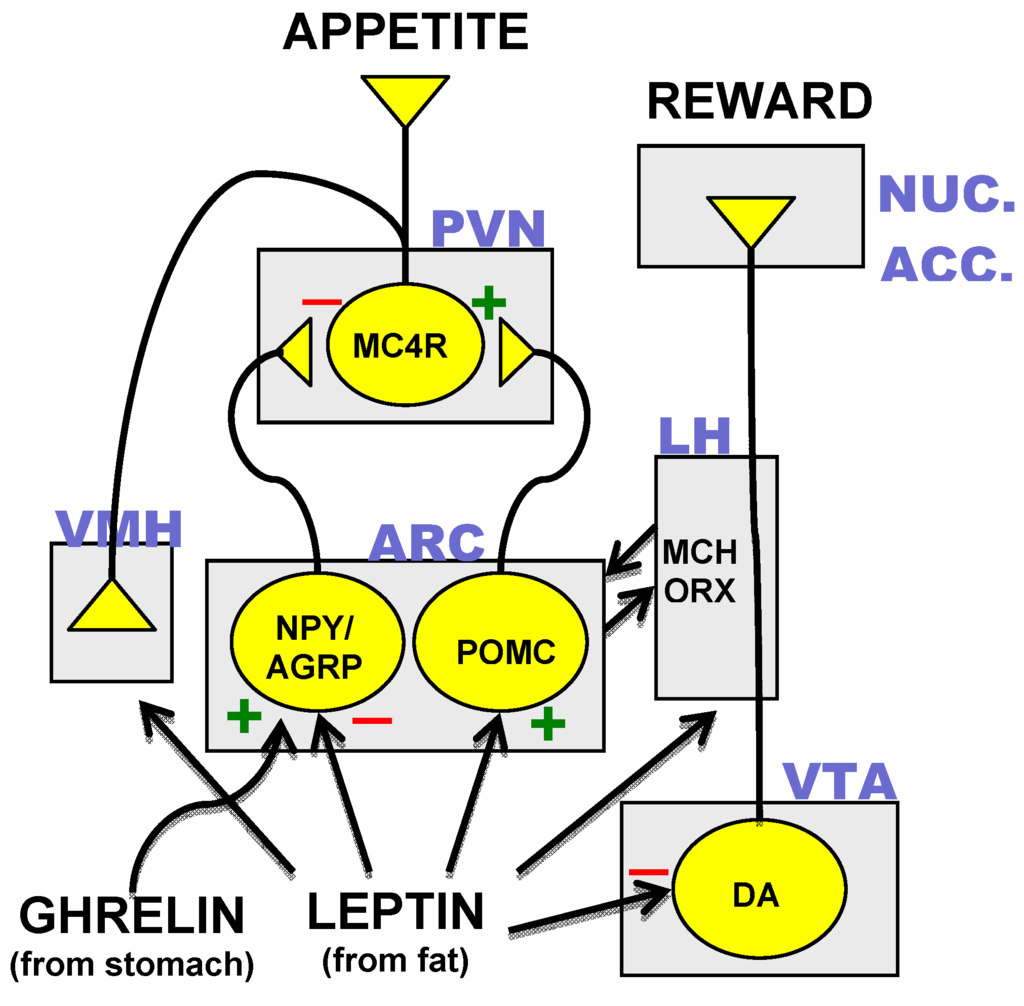 To prevent any repetition of drug rumors related to anti-obesity medications, tesofensine must be meticulously checked and thoroughly studied for its performance and safety and security in dealing with weight-related conditions.
To prevent any repetition of drug rumors related to anti-obesity medications, tesofensine must be meticulously checked and thoroughly studied for its performance and safety and security in dealing with weight-related conditions.On the other hand, rats treated with tesofensine 6 mg/kg and phentermine, which both displayed a lot more stereotypy, were organized in a tiny location but far from the rats in the control and tesofensine 2 mg/kg teams (Fig 7E).
Body weight management attained with lifestyle adjustments, presently accepted anti-obesity medications (AOMs) and bariatric surgical procedure (part a) and connection of drug-induced body weight loss in rodents and people (part b).
Amongst the people who finished 4 years of treatment, the percentage of people who achieved at the very least 5% weight-loss was significantly higher in the orlistat group (52.8%) than in the placebo team (37.3%). At the end of the 4-year study, the advancing incidence of diabetes mellitus was 9.0% in the placebo team and 6.2% in the orlistat team, with a risk reduction rate of 37.3% [17] A. Rats were trained to lick a central spout that dispensed the stimulation a decline of water or services of sucrose. To obtain a benefit (3 drops of water), rats had to choose between 2 lateral spouts. Upper panel reveals the number of tests, and the lower panel the correct efficiency throughout the standard, Tesofensine supplier therapy, and post-tesofensine days. There were no substantial differences in the percent appropriate, the tests per session, or the total quantity consumed between these durations, with the exception of an overall decrease in the variety of trials during the baseline duration as the rat re-learned the job.
When humans were provided amphetamine or sugar pill and called for to preserve constant consuming, the weight-lowering impact was removed (34 ). Later studies in rodents showed that intraperitoneally injected amphetamine is much less reliable in subduing appetite in rats with lateral hypothalamic sores (35 ). In addition, direct hypothalamic shots of amphetamine reduced food intake, and amphetamine activity on the side hypothalamus was hindered by regional management of dopaminergic and β-adrenergic antagonists, and by inhibitors of catecholamine synthesis (36 ).
Ultimately, a high dosage of tesofensine (6 mg/kg) was provided for two days just to stay clear of lethality, which led to boosted mobility and lowered time invested in a silent awake/sleeping state (Fig 7A and 7B). At this high dosage, rats showed clear and robust stereotypy habits with fast onset (Fig 7C and 7D), largely comprising uncontrolled tongue activities and less extreme head swing (S9 Video clip). From a visual evaluation, we note that the stereotypy caused by tesofensine differs a little from that induced by phentermine. However, both medicines share the common attribute of generating unrestrained tongue activities, which earlier studies had stopped working to report.
As a whole, the safety and security profile of Tesofensine side effects is similar to currently approved medicines for the treatment of excessive weight. One of the most frequently reported adverse effects in the overweight populace were dry mouth, migraine, queasiness, insomnia, looseness of the bowels and irregular bowel movements.
Contrave is a combination of bupropion and Tesofensine supplier naltrexone in a sustained-release formulation and is currently in the procedure of resubmission after the FDA declined to approve the medicine in 2011, pointing out security concerns at the time. Naltrexone is an opioid villain and is approved for therapy of alcohol and opioid dependency; it works by obstructing opioid receptors in the brain. It has also revealed effectiveness in therapy of betting problem in addition to alcohol and opioid dependency (Give, Kim, & Hartman, 2008; Give, Odlaug, Potenza, Hollander, & Kim, 2010). Bupropion is currently approved to treat clinical depression as well as smoking cessation and Tesofensine supplier is believed to increase dopamine task in details receptors of the mind. Contrave attained a 6.1% weight-loss at both 28 weeks and 56 weeks of therapy, contrasted to 1.3% of placebo (Aronne et al., 2008; Orexigen Therapies, 2009b). The exploration of leptin in 1994 (ref.47) built our understanding of exactly how peripheral hormones signal to the mind to manage power balance (Box 1; Fig. 2).
